molecules into grams|grams to molecules chemistry : Baguio “Mole is basically the SI unit that represents the quantity of the substance.” For example: If you have one mole of any substance, it means that there are exactly 6.02∗1023atoms or . See more web1 de dez. de 2023 · IO CAPITANO raconte l’incroyable périple de deux jeunes Sénégalais, Seydou et Moussa, qui quittent Dakar pour rejoindre l’Europe. Une odyssée contemporaine à travers les dangers du désert, les périls de la Méditerranée et les ambiguïtés de l’âme humaine. Une narration grandiose qui rappelle notre humanité à l’ordre. Le film .
0 · molecules to grams formula
1 · grams to moles molecules formula
2 · grams to molecules stoichiometry
3 · grams to molecules chemistry
4 · grams to molecules calculator
5 · converting grams to moles
6 · converting grams to molecules
7 · conversion between grams to molecules
8 · More
WEBVídeos Pornôs Com Novinha Gemendo Alto. Mostrar 1-32 de 59. 7:07. Sexo gostoso antes do trabalho. Casal_fuleiro. 26.3K Visualiz. 86% 5:42. NOVINHA GOZANDO NA .
molecules into grams*******“Mole is basically the SI unit that represents the quantity of the substance.” For example: If you have one mole of any substance, it means that there are exactly 6.02∗1023atoms or . See moreFrom the source of Wikipedia: Mole (unit), Nature of the particles, Molar concentration, Standardization, redefinition of SI base units From . See more
According to Centimeter-Gram-Second (CGS) units, the weight of the substance must be determined in grams. This is because every . See more Conversion from grams to molecules requires two conversion factors. First, the molar mass allows you to change mass into mol. Then, knowing that there are 6.022x10^23 molecules in one mol .
molecules into grams grams to molecules chemistry To convert the number of molecules to moles, we divide the number of molecules by the Avogadro's number. To convert moles to grams, we multiply the number of moles by the molar mass, which can.
grams to molecules chemistry This chemistry video tutorial explains how to convert grams to molecules. it also explains the conversion of molecules to grams which is useful in common .Convert moles to grams with precision using our Moles to Grams Calculator. Simplify your chemistry calculations for accurate substance mass conversions
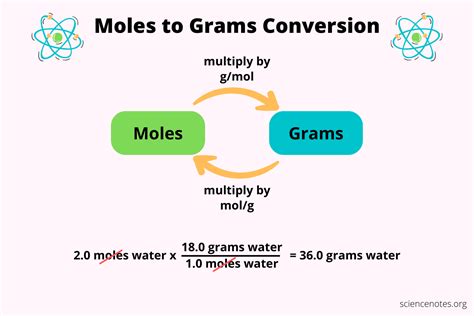
Grams from moles. Moles: Formula Weight (daltons): Grams =. Analyze, graph and present your scientific work easily with GraphPad Prism. No coding required. Try for Free. We’ll use the Mole Map as our guide to setting up and understanding how to convert between moles, grams, liters, and particles (molecules, atoms .). Here are the steps for converting moles to grams: Start with the number of moles and the chemical formula of the substance. Find the molar mass of the substance. Add together the atomic mass of each .
One mole of isotopically pure carbon-12 has a mass of 12 g. For an element, the molar mass is the mass of 1 mol of atoms of that element; for a covalent molecular compound, it is the mass of 1 mol of . To simply convert from any unit into grams, for example, from 5 kilograms, just multiply by the conversion value in the right column in the table below. 5 kg * 1000 [ (g) / (kg) ] = 5000 g. To convert from g .Carrying out grams to moles conversion: n = m M. n = 5000 90.075. n = 55.503 m o l e s. You can also verify the results by putting the values in free grams to molecules calculator. Example # 02: Consider you have 2.3 grams of NaCl.
The molar mass of KClO3 is 122.548 g/mol. Multiply the given number of moles (2.50 mol) by the molar mass (122.548 g/mol) to get the grams. The number of grams of KClO3 will be 306.37. And here is how you should enter this problem into the calculator above: moles to grams problem solution.Quick conversion chart of moles O2 to grams. 1 moles O2 to grams = 31.9988 grams. 2 moles O2 to grams = 63.9976 grams. 3 moles O2 to grams = 95.9964 grams. 4 moles O2 to grams = 127.9952 grams. 5 moles O2 to grams = 159.994 grams. 6 moles O2 to grams = 191.9928 grams. 7 moles O2 to grams = 223.9916 grams. 8 moles O2 to .
This conversion factor can be found on the line between the starting and destination boxes in the figure (in this case, it's the molar mass of NH 3 ). 63 g NH3 × 1 mol NH3 ⁄ 17 g NH3. Multiply these numbers together, making sure to cancel out any appropriate units. 63 g NH 3 × 1 mol NH3 ⁄ 17 g NH3 = 3.7 mol NH 3. Molar mass = (2 x 1.008) + (2 x 15.999) Molar mass = 34.014 grams/mol. Multiply the molar mass by the number of moles to get the grams: grams of hydrogen peroxide = (34.014 grams/mol) x (0.700 mol) = 23.810 grams. Answer. There are 23.810 grams of hydrogen peroxide in 0.700 moles of hydrogen peroxide.Grams from moles. Moles: Formula Weight (daltons): Grams =. Analyze, graph and present your scientific work easily with GraphPad Prism. No coding required.One mole consists of Avogadro number of atoms. If you know the quantity of mole, it can be converted into grams and vice versa. The formula for moles to grams is given by. Example 1 – Calculate the mass in grams of 3.6 mol of H 2 SO 4. Solution. Look for the atomic masses of hydrogen, sulfur and oxygen. H = 1.008. S = 32.06. O = 16

Learn a SIMPLE way to convert the number of molecules of a covalent compound to mass of that covalent compound in the Mole Conversion II Practice Problem The molar amount of a substance may be calculated by dividing its mass (g) by its molar mass (g/mol): The factor-label method supports this mathematical approach since the unit “g” cancels and the answer has units of “mol:”. 4.7 g K ⎛⎝ mol K 39.10 g K ⎞⎠ = 0.12mol K 4.7 g K ( mol K 39.10 g K) = 0.12 mol K.Quick conversion chart of moles Co2 to grams. 1 moles Co2 to grams = 117.8664 grams. 2 moles Co2 to grams = 235.7328 grams. 3 moles Co2 to grams = 353.5992 grams. 4 moles Co2 to grams = 471.4656 grams. 5 moles Co2 to grams = 589.332 grams. 6 moles Co2 to grams = 707.1984 grams. 7 moles Co2 to grams = 825.0648 grams.
Exercise 4.10.1 4.10. 1. Consider a problem that requires a calculation of the mass of molecular nitrogen that contains 7.2153 × 10 24 molecules of molecular nitrogen. The molecular weight of molecular nitrogen is 28.02 g/mol N 2. Identify the indicator information in the given problem, and state which molar standard is associated with each .To convert 8.00 x 1019 molecules of HCN to grams, you first need to understand Avogadro's number, which is the number of particles in one mole of substance: 6.022 x 1023 molecules/mol. Using this information, you can calculate the number of moles of HCN: Number of moles = (8.00 x 1019) / (6.022 x 1023) moles.A Percent to Grams Calculator is a handy tool for converting percentages into gram values, especially when dealing with solutions, mixtures, or substances where the quantity of a particular component is expressed as a percentage of the total weight or volume. . Grams = (Percentage / 100) × Total Weight or Volume. Where: Grams is the quantity .
Quick conversion chart of moles Co2 to grams. 1 moles Co2 to grams = 117.8664 grams. 2 moles Co2 to grams = 235.7328 grams. 3 moles Co2 to grams = 353.5992 grams. 4 moles Co2 to grams = 471.4656 grams. 5 moles Co2 to grams = 589.332 grams. 6 moles Co2 to grams = 707.1984 grams. 7 moles Co2 to grams = 825.0648 grams.
Exercise 4.10.1 4.10. 1. Consider a problem that requires a calculation of the mass of molecular nitrogen that contains 7.2153 × 10 24 molecules of molecular nitrogen. The molecular weight of molecular nitrogen is 28.02 g/mol N 2. Identify the indicator information in the given problem, and state which molar standard is associated with each .To convert 8.00 x 1019 molecules of HCN to grams, you first need to understand Avogadro's number, which is the number of particles in one mole of substance: 6.022 x 1023 molecules/mol. Using this information, you can calculate the number of moles of HCN: Number of moles = (8.00 x 1019) / (6.022 x 1023) moles.A Percent to Grams Calculator is a handy tool for converting percentages into gram values, especially when dealing with solutions, mixtures, or substances where the quantity of a particular component is expressed as a percentage of the total weight or volume. . Grams = (Percentage / 100) × Total Weight or Volume. Where: Grams is the quantity .
This online calculator converts to moles given grams and converts to grams given moles All online calculators Articles Suggest a calculator . This number is the number of molecules of a specific compound in which when you multiply the compound by it, it converts atomic mass into grams. For example, one mole of hydrogen gas ( #H_2# ) or #6.022*10^23# molecules of #H_2# weighs 2.016 grams because one molecule of #H_2# has an atomic weight of 2.016.Quick conversion chart of moles Cl2 to grams. 1 moles Cl2 to grams = 70.906 grams. 2 moles Cl2 to grams = 141.812 grams. 3 moles Cl2 to grams = 212.718 grams. 4 moles Cl2 to grams = 283.624 grams. 5 moles Cl2 to grams = 354.53 grams. 6 moles Cl2 to grams = 425.436 grams. 7 moles Cl2 to grams = 496.342 grams. 8 moles Cl2 to grams = . We can combine the two types of problems into one. Mass and number of particles are both related to grams. In order to convert from mass to number of particles or vice-versa, a conversion to moles is required. Figure [Math Processing Error]: Conversion from number of particles to mass, or from mass to number of particles requires two steps. A mole is 6.022 * 1023 things, whereas a dozen is 12 things. So this is where we get the conversion between 1 mole and 6.022 * 10 23 molecules. 6.022 * 10 23 molecules. 1 mol. or. 1 mol. 6.022 * 10 23 molecules. This ratio is seen on the right arrow (the green text) in the conversion map below. On a side note, I tend to abbreviate the .
It measures a certain quantity of entities (atoms or molecules) that is equivalent to 6.022 x 10^23, or Avogadro's number. . Now that we've established the importance of moles to grams conversion, let's dive into the practical aspect of using the calculator effectively. Step 1: Input Moles. Let's do a quick example to help explain how to convert from moles to grams or grams to moles. We know we have 10 g of HCl, which has a molecular weight of 36.5 g/mol. Let's plug these numbers into the above equation: mole = 10 / 36.5 = 0.27 moles = 1.626×10²³ molecules of HCl
How to Convert Moles to Grams. Grams and moles are both units used in chemistry to measure matter in different ways. A gram is an SI unit of measurement for mass equal to 1/1,000 of a kilogram, and the mole is the SI base unit used to represent the quantity of a substance.. One mole is equal to 6.02214076 × 10 23 elementary units of matter, such .
Resultado da Live betting and app. Available. Sport type for bets. Football, basketball, soccer, tennis, baseball, hockey and more. Deposit Options. VISA cards, MuchBetter, MasterCard, Paysafecard and more. 888sport betting odds delivers the best of the latest sports coverage of NFL odds, NBA odds, .
molecules into grams|grams to molecules chemistry